Dr. Daniel Stone
University of Leeds, UK
Background
Dr. Daniel Stone’s research group, from the University of Leeds in the United Kingdom, investigates oxidation processes in atmospheric and combustion chemistry. Dr. Stone is particularly interested in the chemistry of reactive species such as OH, HO2, and Criegee intermediates (R2COO) that control atmospheric composition and fuel combustion. His research requires a combination of laboratory experiments, field measurements, and numerical modelling.
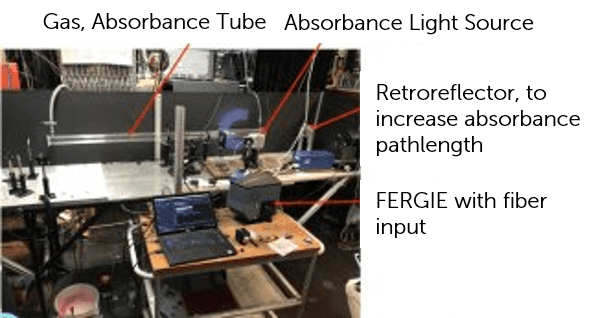
Figure 1: Absorbance spectroscopy experimental set up with FERGIE system attached.
Challenge
Past laboratory experiments conducted by Dr. Stone investigated the kinetics of the CH2OO Criegee intermediate. These experiments provided the first direct measurements of CH2OO reaction kinetics as a function of pressure, obtained by monitoring the HCHO reaction products by laser-induced fluorescence spectroscopy (Stone et al., 2014). This work also indicated significant yields of CH2OO following photolysis of CH2I2 in the presence of O2 under atmospheric conditions (Stone et al., 2013), which impacts the understanding of oxidation chemistry in iodine-rich coastal regions.
Since then, Dr. Stone’s research group has focussed on the development of a quantum cascade laser (QCL) infrared absorption experiment to monitor Criegee intermediates directly under atmospheric conditions, and to monitor the production of SO3 in reactions of Criegee intermediates with SO2. These experiments enable the assessment of the atmospheric impacts of Criegee chemistry on production of sulfuric acid and sulfate aerosol, and thus the impact on air quality and climate change.
Once FERGIE was integrated into the experiment setup, I was free to determine the triggering and able to produce relevant time-dependent data within a single measurement day.
Solution
Dr. Stone designed an experiment with the FERGIE system (previous version of the IsoPlane 81) to measure the transient absorption of a gas/gas mixture after flash photolysis using a high-power laser pulse. By readily connecting a fiber to the FERGIE fiber port in the experimental set up, the trigger input of FERGIE could be utilized to sync acquisition with an external delay generator.
By utilizing the FERGIE’s spectra kinetrics mode (with a window hight of 50 rows) a time escale of ~290 µsec per spectrum was achieved. This reduced the timescale of experiments by a factor of 5-6x, just by reducing the spectra kinetics window height. This enabled detection of fast changes in the absorbance, on the msec to sub-msec scale. Sensitivity was increased by repeating the experiment 100 times.

