Fluorescence, Phosphorescence and Photoluminescence Spectroscopy
Fluorescence, phosphorescence, and photoluminescence occur when a sample is excited by absorbing photons and then emits them with a characteristic decay time. Fluorescence is when the absorbing and emitting species is an atom or molecule. Phosphorescence is similar to fluorescence but with a longer time gap between absorption and emission. Photoluminescence is the term physicists use to describe the absorption and emission of light by materials such as semiconductor and nanotubes.
A chemical species absorbs a photon and is excited to a singlet electronic state. It then relaxes via non-radiative mechanisms and emits a lower-energy photon. This allows it to transition to the ground electronic state. A spectrograph and camera can be used to obtain the spectrum of emission, with the intensity correlating to the concentration of the chemical species.

Application Notes
High-Sensitivity, Large-Format CCD Camera -Enable Multidimensional Characterization of Soil-Grown Root Systems
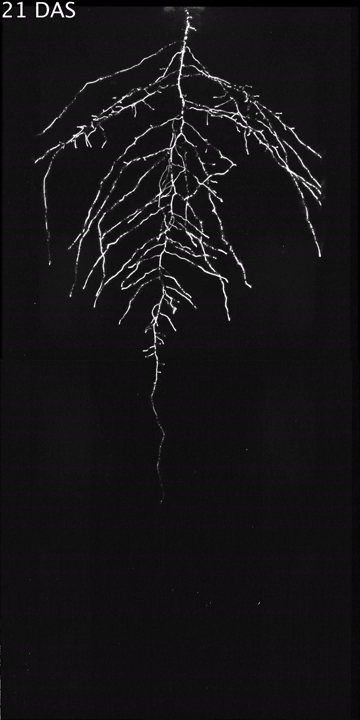
Recently, a team of scientists working in North America and Europe developed an innovative growth and imaging platform, known as GLO-Roots, that allows root architecture and gene expression to be studied in soil-grown plants.
GLO-Roots (Growth and Luminescence Observatory for Roots; U.S. patent application: 13/970,960) is a collaborative effort between…Read Full Article
Technical Notes
A New Dawn for NIR Spectroscopy
Featuring two revolutionary back-illuminated deep-depletion sensors, Teledyne Princeton Instruments BLAZE cameras for spectroscopy provide the highest near-infrared quantum efficiency, fastest spectral rates, and deepest thermoelectric cooling available in a CCD platform. Lower thermally generated dark noise, combined with …Read Full Article

