Introduction
One of the main areas of research at the Memorial Sloan Kettering Cancer Center in New York City is the development of nanoscale sensors to detect cancer at its earliest stages. The research group led by Dr. Daniel Heller uses novel nanomaterials with unique optical properties, making it easier to identify disease biomarkers within the body and thus permit detection before symptoms arise. These nanotechnologies also allow the measurement of important molecules within live cells and tissues, offering new tools that accelerate biomedical research [1].
Working in the near-infrared (NIR) region of the spectrum affords several advantages, such as the abilities to circumvent unwanted fluorescence backgrounds and to probe more deeply into sample surfaces. Over the past few years, the advent of deep-cooled cameras that employ indium gallium arsenide (InGaAs) focal plane arrays (FPAs) has increased the utility of various NIR spectroscopy and imaging techniques for low-light-level scientific applications [2].
This type of scientific camera can be even more helpful to researchers when used in concert with a new class of dispersive spectrographs that feature an innovative Schmidt-CzernyTurner (SCT) design. High-precision SCT spectrographs greatly reduce optical aberrations, providing sharp images with superb spatial resolution across the entire focal plane and enabling researchers to utilize the full two dimensions of the FPA sensor to acquire images [3].
Dr. Heller’s research group in New York has successfully used such light-dispersion and -detection tools to perform novel experiments investigating properties of photoluminescent (PL) single-walled carbon nanotubes (SWCNTs), which could ultimately result in the development of new optical probes and sensors for biophysical measurements and biomedical applications [4-6]. This application note will present some of the highlights of the group’s work as well as the integral role played by advanced SCT spectrographs and deep-cooled InGaAs FPA cameras.
Example of Experimental Setup
Dr. Heller’s research group has performed several PL spectroscopy studies on SWCNTs in recent years. Three of these studies will be highlighted in this note.
The first study, reported in 2014, involved the encapsulation of SWCNTs with a diverse set of functional coatings (polymers) that exhibited ordered surface coverage on the carbon nanotubes and allowed systematic modulation of nanotube optical properties [4]. In the second study, the researchers used the intrinsic NIR emission of semiconducting SWCNTs to optically reconstruct the carbon nanotubes’ localization within a three-dimensional volume and thus resolve the relative permeability of two different multicellular tumor spheroids [5]. Finally, in the third study, SWCNT emission energy response to solution ionic potentials was investigated and it was observed that nanotubes respond to cell surface electrostatic potentials that are mediated by membrane proteins [6].
Although each of these SWCNT studies necessitated the implementation of distinct experimental protocols, all three used a next-generation Princeton Instruments IsoPlane®-320 spectrograph and a deep-cooled Princeton Instruments NIRvana® InGaAs FPA camera to perform near-infrared photoluminescence spectroscopy. For example, in Study #3, the researchers integrated the IsoPlane and the NIRvana into their own custom-built apparatus to enable PL excitation/emission spectroscopy of nanotubes on live eukaryotic cells (see Figure 1).

When investigating SWCNT emission energy response to solution ionic potentials (Study #3) with the experimental setup shown in Figure 1, the sample was excited using a supercontinuum light source coupled to a variable bandpass filter in order to tune the excitation from 500 to 827 nm with a 20 nm bandwidth. The light was injected into an inverted fluorescence microscope through a 50X objective. The same objective was utilized to collect the resultant NIR emission and direct it into the IsoPlane spectrograph, which was coupled to the NIRvana camera (thermoelectrically cooled InGaAs array: 640 x 512 pixels; pixel size: 20 x 20 μm; quantum efficiency: >85% in the 0.9–1.7 μm range) [6].
To conduct excitation/emission measurements for Study #3, the excitation was varied from 500 to 827 nm in steps of 3 nm. At each excitation wavelength, with an exposure time of 0.5–3.0 sec, the emission from 930 to 1370 nm was dispersed using a ruled grating (86 grooves/mm). Corrections for wavelength-dependent variations in excitation power (5–30 mW measured at the sample), as well as grating and detector efficiencies, were applied. The system was automated to illuminate the sample with 109 different excitation bands and collected spectra from carbon nanotubes in solution or in contact with a cell monolayer to produce a full photoluminescence plot in 0.5–5 min [6].
Data & Results
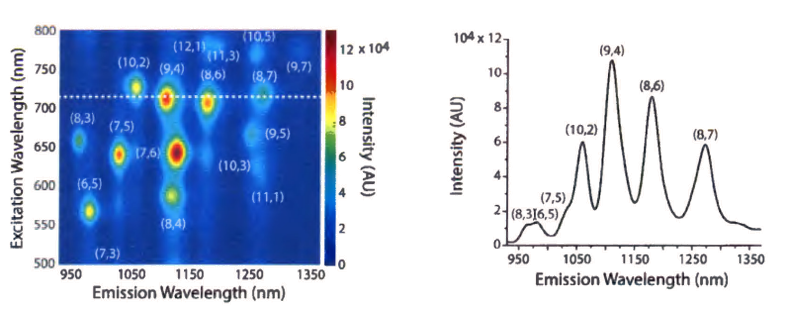
Study #1 (Polymer-Coated SWCNTs): In this study, the astigmatism-free IsoPlane spectrograph and the deep-cooled InGaAs camera were utilized to perform PL excitation/emission measurements on polycarbodiimide−SWCNTs. The excitation wavelength was varied from 491 to 824 nm and the emission was recorded from 915 to 1354 nm (see Figure 2).

emission profiles of polycarbodiimide−SWCNTs and surfactant-suspended SWCNTs. Data courtesy of Dr. Daniel Heller, Memorial Sloan Kettering Cancer Center, New York. First published in J. Budhathoki-Uprety, P.V. Jena, D. Roxbury, and D.A. Heller, “Helical polycarbodiimide cloaking of carbon nanotubes enables inter-nanotube exciton energy transfer modulation,” J. Am. Chem. Soc. 136 (44), 15545–15550 (2014).
The researchers reported noncovalent functionalization of SWCNTs via encapsulation in helical polycarbodiimides to form water-soluble, well-dispersed, polymer–nanotube complexes with NIR emission that were stable under ambient conditions. The polymers facilitated the intensity modulation of nanotube fluorescence and enabled inter-nanotube exciton energy transfer between individually encapsulated nanotubes. This was the first instance of exciton energy transfer produced spontaneously between nanotubes due to Coulombic attraction between the encapsulating polymers and it displayed directed reversibility. The finding augurs the measurement of dynamic processes and a potential mechanism for switchable molecular probes and sensors [4].
Study #2 (Tumor Permeability): Here, the intrinsic NIR fluorescence of SWCNTs was leveraged to interrogate the permeability of multicellular tumor spheroids. The Teledyne Princeton Instruments IsoPlane spectrograph and InGaAs camera were employed to perform excitation/emission measurements on surfactant-sodium-deoxycholate−SWCNTs (see Figure 3).
The research group reported developing a tumor spheroid model of murine liver cancer cells. These tumor spheroids were compared with a breast-cancer cell line that forms spheroids under low-adhesion conditions. Widefield NIR fluorescence microscopy in live cells spatially resolved the locations of nanotubes associated with multicellular tumor spheroids. The
researchers found that the nanotubes showed little penetration in one type of spheroids (the liver cancer), but penetrated to the center of the other (the breast cancer). Therefore, the group effectively presented the use of NIR-fluorescent SWCNTs as a validated and qualitative method to interrogate the permeability of live tumor spheroids [5],
Study #3 (Live Cell Membranes): In this study, the spectrograph and InGaAs camera were utilized to perform excitation/emission spectroscopy of SWCNTs associated with the membranes of live cells (see Figure 4). As mentioned in the “Example of Experimental Setup” section, the excitation was varied from 500 to 827 nm in steps of 3 nm. At each excitation wavelength, with an exposure time of 0.5–3.0 sec, the emission from 930 to 1370 nm was dispersed using a ruled grating with 86 grooves/mm.

The researchers found that the nanotube photon emission energy responded to charge accumulation mediated by cell surface proteins and that nanotube photon emission energy correlated with both the degree to which a cell adheres to a substrate, as well as the whole-cell zeta potential. They asserted that the photoluminescence responses on the cell surface could be recapitulated in vitro by introducing ionic charge into the local environment of the nanotube. The research group also proposed a mechanism in which the nanotube photoluminescence is modulated by the charge density on live cell surfaces. This study suggested that the nanotube optical bandgap modulation can be mediated by ionic or polyelectrolyte charge accumulation on the nanotube surface. The findings portend a nanoscale tool for the optical quantification of electrostatic charge accumulation on live cell membranes for biomedical applications [6].
Enabling Technology
The aforementioned research relied on the award-winning IsoPlane-320 spectrograph (see Figure 5) from Teledyne Princeton Instruments. This high-precision instrument’s unique optical design completely eliminates field astigmatism at all wavelengths and at all points across the focal plane. Coma is reduced to negligible levels. Reduced optical aberrations result in significantly improved signal-to-noise ratio (SNR) and exceptional image quality. IsoPlane spectrographs feature a 320 or 160 mm focal length and a three-position, on-axis grating turret.

In addition to an IsoPlane-320 spectrograph, Dr. Heller’s group utilized an NIR-sensitive InGaAs FPA camera from Princeton Instruments to conduct the research highlighted herein. This camera, the NIRvana:640 (see Figure 6), differentiates itself from other InGaAs cameras via a number of scientific performance features, including deep cooling, low dark noise, high linearity, low read noise, high frame rates, intelligent software, and precision control over integration times.

First and foremost, either maintenance-free thermoelectric cooling or liquid nitrogen can be employed to chill the NIRvana’s two-dimensional 640 x 512 InGaAs FPA detector down as low as -85°C or -190°C, respectively. A proprietary cold shield design and vacuum technology facilitates the lowest possible dark noise, which helps increase sensitivity as well as preserve SNR for long exposure times.
A thermoelectrically cooled NIRvana camera offers the ability to expose from 2 μsec up to many minutes, whereas the exposure time of an LN-cooled NIRvana camera can range from 100 μsec up to 1 hour. Ultra-low-noise readout electronics help ensure good SNR even when the camera is operated at its maximum full-frame readout rate (i.e., 110 full fps for a thermoelectrically cooled NIRvana; 2.77 full fps for an LN-cooled NIRvana). Excellent system linearity means that every NIRvana camera is highly reliable for scientific research.
Furthermore, Princeton Instruments’ 64-bit LightField® data acquisition software, available as an option, provides a powerful yet easy-to-use interface that puts real-time online processing capabilities at the researcher’s fingertips. NIRvana cameras can be integrated into larger experiments using an available National Instruments LabVIEW® toolkit. Full triggering support is provided for synchronization with external equipment.
Acknowledgements
Princeton Instruments would like to thank Dr. Daniel Heller, Memorial Sloan Kettering Cancer Center, for his invaluable contributions to this application note.
Resources
Additional details about the work being performed by Dr. Daniel Heller’s research group can be found by visiting: https://www.mskcc.org/research-areas/labs/daniel-heller
References
- https://www.mskcc.org/research-areas/labs/daniel-heller [accessed online in May 2016]
- Introduction to scientific InGaAs FPA cameras. Princeton Instruments Technical Note (2012).
- Better imaging with a Schmidt-Czerny-Turner spectrograph. Princeton Instruments Technical Note (2013).
- Budhathoki-Uprety, P.V. Jena, D. Roxbury, and D.A. Heller, “Helical polycarbodiimide cloaking of carbon nanotubes enables inter-nanotube exciton energy transfer modulation,” J. Am. Chem. Soc. 136 (44), 15545–15550 (2014).
- V. Jena, Y. Shamay, J. Shah, D. Roxbury, N. Paknejad, and D.A. Heller, “Photoluminescent carbon nanotubes interrogate the permeability of multicellular tumor spheroids,” Carbon 97, 99–109 (2016).
- Roxbury, P.V. Jena, Y. Shamay, C.P. Horoszko, and D.A. Heller, “Cell membrane proteins modulate the carbon nanotube optical bandgap via surface charge accumulation,” ACS Nano 10, 499–506 (2016).