Advanced Instrumentation Enables Improvements in High-Speed, Label-Free Imaging
Overview
Popular molecular imaging techniques are only able to reveal the distribution or behavior of specific molecules within the human body that have been labeled with pigments or fluorescent proteins. Raman spectroscopy, however, allows researchers to identify the components of unlabeled molecules via spectral analysis. A vibrational (Raman) spectrum is therefore commonly referred to as a molecular fingerprint. The use of such label-free molecular imaging increases the likelihood that unexpected changes and abnormalities in the body will be discovered.1
One innovative Raman-based technique, known as ultra-multiplex CARS spectroscopic imaging, demonstrates great utility for miniaturized, non-invasive, real-time, and cellular-level molecular diagnostics in experimental and clinical applications. This technique, which enables biological structures and processes to be observed and analyzed via quantitative photonic data collected simultaneously across a broad spectral range, continues to be refined and improved.
For example, Associate Professor Hideaki Kano of the University of Tsukuba, Japan, and his colleagues recently performed ultra-multiplex CARS spectroscopic imaging of living cells (18 colors corresponding to 18 wavenumbers) with an effective exposure time per pixel of 1.8 msec, the fastest speed reported to date for broadband CARS whose spectral coverage is ~3000 cm-1.2
CARS Basics
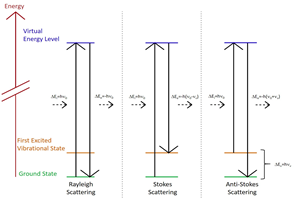
Coherent anti-Stokes Raman scattering (CARS) derives its name from the fact that rather than using a traditional single laser this nonlinear spectroscopy technique utilizes a pair of very strong coherent laser beams to irradiate a sample, thereby producing an anti-Stokes frequency signal. Whereas the first laser’s frequency is usually constant, the frequency of the second can be tuned so that the frequency difference between the two lasers equals the frequency of a Raman-active mode of interest. This particular mode will be the only extremely strong peak in the Raman signal.3
CARS is orders of magnitude stronger than normal Raman emission. A monochromator is not necessarily required to perform CARS; a wideband interference filter with a detector placed behind the filter may work instead. Mathematical and schematic descriptions of CARS are presented next. See Figure 1a.
Two laser beams with frequencies ω1 and ω2 (ω1 > ω2 ) interact coherently to produce a strong scattered light with frequency 2ω1 – ω2 . If the frequency difference between the two lasers (ω1 – ω2 ) equals the frequency ωm of a Raman-active vibrational, rotational, or other mode, then a strong light with frequency ω1.
In other words, to obtain a strong Raman signal, the second laser frequency should be tuned in such a way that ω2 = ω1 – ωm. Then the frequency of strong scattered light will be 2ω1 – ω2 = 2ω1 – (ω1 – ωm) = ω1 + ωm, which is higher than the excitation frequency ω1.

In addition to overcoming the low signal intensities of most biomolecules, CARS provides directional emission and narrow spectral bandwidth, without interference from autofluorescence. CARS studies include microbial cells, eukaryotic cells, trapped cells, medical tissue, epithelial tissue, muscle tissue, nervous tissue, lung tissue, breast tissue, bone, and skin.4
The rapid scanning capability of CARS microscopy is invaluable to researchers investigating real-time dynamics. Furthermore, as opposed to fluorescence microscopy techniques, cells can be repeatedly imaged in CARS without the fading problems attributable to photobleaching.5
Multiplex CARS and Ultra-Multiplex CARS
Multiplex CARS (see Figure 1b) is an enhanced CARS technique that employs a multichannel detector (e.g., a scientific CCD or CMOS camera) coupled to a spectrometer in order to cover the broad spectral range of the coherent Raman signal.
By using a broadband laser source (e.g., a supercontinuum light source or a femtosecond laser source) instead of the tunable laser ω2, the typical spectral coverage of the multiplex CARS signal can be further extended, reaching approximately 3000 cm-1. This ultra-multiplex CARS spectroscopic imaging approach is broad enough to detect all vibrational fundamental modes — including the critically important fingerprint region (in which unknown organic compounds can be identified as well as distinguished from one another) along with the C-H and O-H stretching regions (useful for identifying the rough molecular distribution of lipids, proteins, and nucleic acids).2
New Ultra-Multiplex CARS Experiment
When Dr. Hideaki Kano attended the CLEO Conference in San Francisco as a doctoral student back in the spring of 2000, he was attracted to supercontinuum generation using photonic crystal fibers — which require only a femtosecond laser oscillator to generate a supercontinuum (SC). Later, after beginning his academic career in the Hamaguchi Lab at the University of Tokyo, he developed a home-built inverse Raman (now frequently referred to as a stimulated Raman loss, or SRL) spectroscopic system utilizing SC generation.6
During his experiment, Dr. Kano happened to find the CARS signal, which was very intense and actually much easier to detect than the inverse Raman (i.e., SRL) signal. He quickly realized that combining SC generation with a spectroscopic apparatus would be a breakthrough not only in the field of fundamental spectroscopy but in the life sciences as well, where spectroscopic imaging was just emerging.
Experimental Setup
Two laser sources were incorporated in Dr. Kano’s experimental setup, as were a new highreadout- speed CCD camera with ultrahigh NIR sensitivity and a high-throughput spectrometer. See Figure 2.
The first laser source, which was based on a master oscillator fiber amplifier (MOFA) configuration, involved a microchip oscillator — a passively Q-switched laser comprising an Nd:YVO4 crystal bonded with a saturable absorber mirror — and a Yb-doped fiber amplifier. The second source was a passively Q-switched microchip Nd:YAG laser. The researchers were able to switch the laser sources based on the type of sample being studied, affording good experimental flexibility in terms of wavelength, temporal duration, repetition rate, and output average power.2
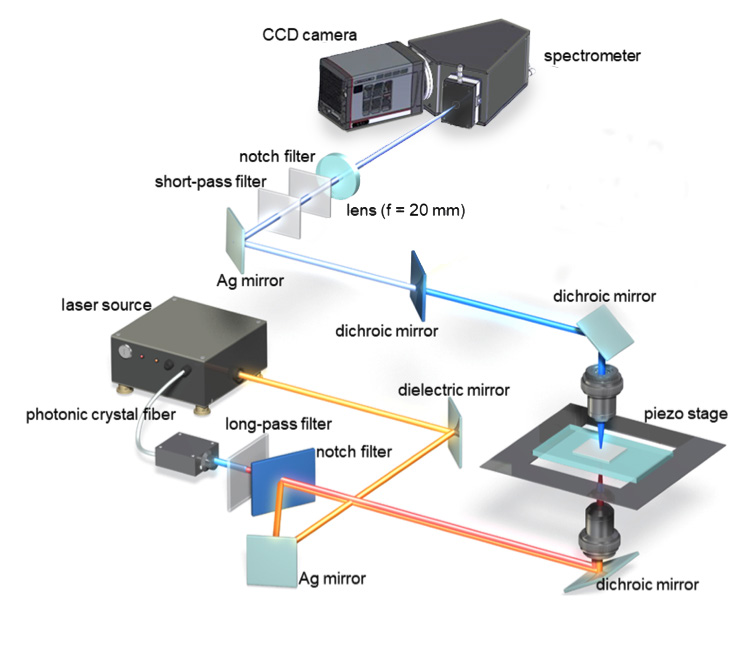
A newly available scientific CCD camera provided significantly higher NIR sensitivity than that afforded by the state-of-the-art CCD camera used by Dr. Kano and his associates during their previous ultra-multiplex CARS spectroscopic imaging study of living cells.7
As well as its greater sensitivity at important NIR in vivo imaging wavelengths, the new camera delivered the high-speed operation needed to synchronize with the ultrafast lasers used in Dr. Kano’s time-resolved studies. The researchers coupled the camera to a high-throughput spectrograph, which dispersed the lens-collected CARS signal for detection by the camera.
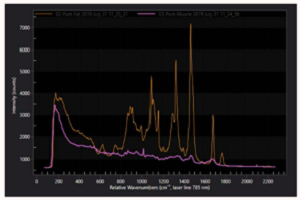
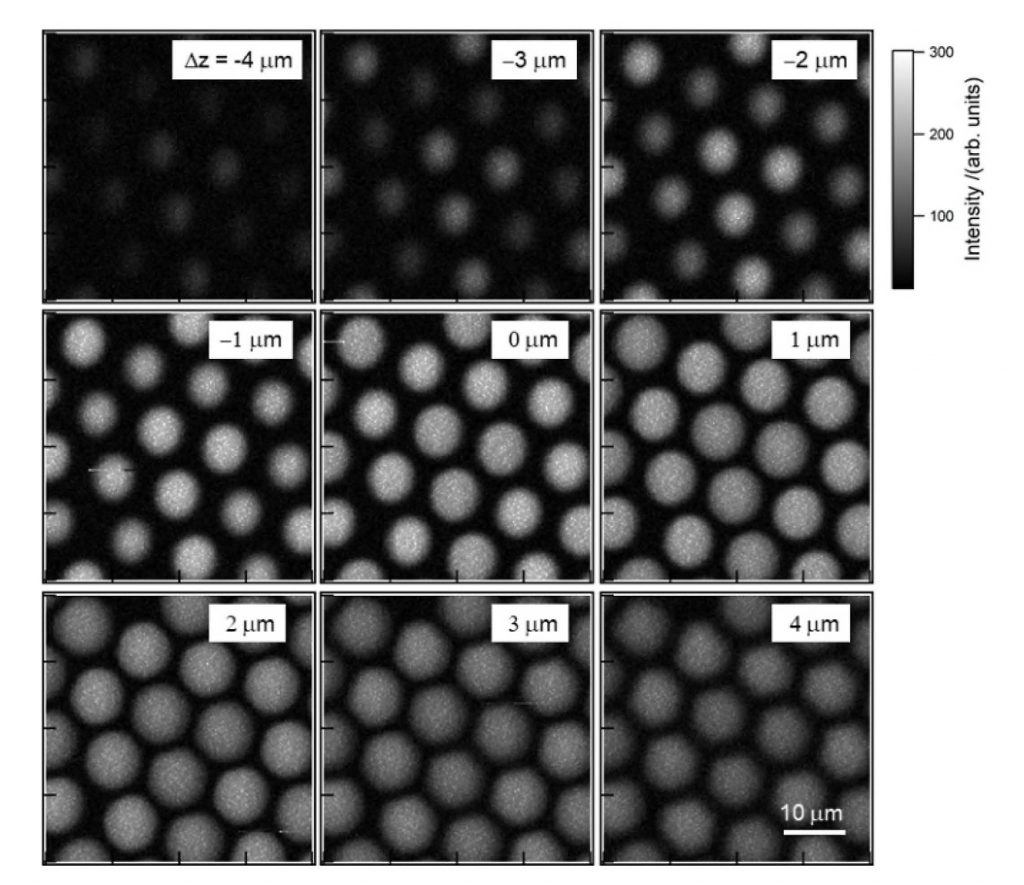
Before being utilized to image living cells, the experimental system was first evaluated by measuring the CARS signal of a polystyrene bead (diameter: 10 μm). The ultra-multiplex CARS signal of the bead was detected in the range from 600 cm-1 to 3600 cm-1 with resolution <10 cm-1. The pixel dwell time was ~1 msec and CARS images of 161 × 161 pixels were acquired of the beads with a total data-acquisition time of ~28 sec (Figure 3).2
Imaging of Live A549 Cells
After verifying the performance of the system by imaging polystyrene beads, the researchers imaged a living cell (A549) using the high-speed, high-sensitivity CCD camera and the MOFA laser source (Figure 4). The sharp peak observed at 2850 cm-1 on the spectrum rendered in red corresponds to one of the bright spots inside the cell, which in turn corresponds to the CH2 stretching vibrational mode observed primarily in intracellular lipid droplets. Note that a raw CARS signal consists of a vibrationally resonant signal and a non-resonant background. These two components interfere with each other and produce dispersive line shapes.2
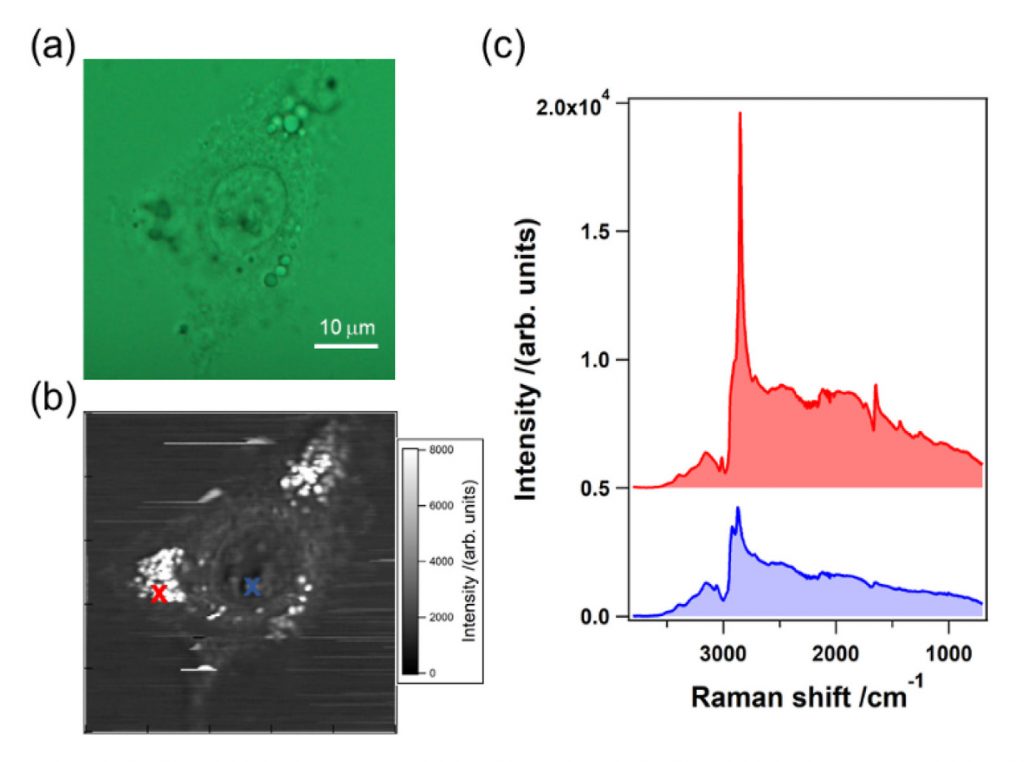
Dr. Kano and his colleagues next extracted the pure vibrationally resonant signal to obtain spontaneous-Raman-equivalent spectral profiles by performing numerical analysis. They found the main features of these resultant spectral profiles to be in good agreement with those of intracellular lipids and proteins, respectively. More than a dozen characteristic Raman bands between 3427 cm-1 and 1009 cm-1 corresponded to the vibrational modes.2
For additional data and a more detailed discussion of this study, please refer to the following article: Hideaki Kano, Takumi Maruyama, Junko Kano, Yuki Oka, Daiki Kaneta, Tiffany Guerenne, Philippe Leproux, Vincent Couderc, and Masayuki Noguchi, “Ultramultiplex CARS spectroscopic imaging with 1-millisecond pixel dwell time,” OSA Continuum 2, 1693–1705 (2019).
Progress and Outlook
The researchers performed ultra-multiplex (600 cm-1 – 3600 cm-1) CARS spectroscopic imaging on living cells, reporting the fastest time to date for broadband CARS whose spectral range is ~3000 cm-1. Based on the clear molecular fingerprint, Dr. Kano and his colleagues visualized intracellular molecular distribution with more than 15 vibrational bands. The exposure time of 1 msec on software equated to an effective exposure time per pixel of ~1.8 msec, a significant reduction when compared to Dr. Kano’s previous study on living-cell imaging (in which the exposure time per pixel was 50 msec). The improvement was mainly attributable to the higher sensitivity and readout speed of the newly available CCD camera.2,7
The combination of this high-speed technique and multivariate analysis methods can help life scientists and medical doctors gain meaningful insights into the dynamics of intracellular metabolic activity. Dr. Kano’s research group is currently collaborating with a pathologist to develop a new spectroscopic diagnostic tool using the molecular fingerprint.
Key Technologies
To facilitate their most recent work, the researchers selected a Teledyne Princeton Instruments BLAZE® camera with a proprietary “super-deep-depletion” CCD manufactured from high-resistivity bulk silicon8. Designed to yield the highest near-infrared quantum efficiency of any silicon device available, the camera featured a back-illuminated 1340 x 400 spectroscopic array format (20 μm square pixels) capable of being cooled down to -95°C in air, without chillers or liquid assist, for low-dark-current performance.
BLAZE also offered the researchers the fastest ADC speeds available in a CCD camera. The new sensor platform’s dual 16 MHz readout ports were engineered to enable unprecedented spectral rates of more than 1600 spectra/second with full vertical binning and up to 215 kHz when operated in kinetics mode.
The spectrograph used in concert with the BLAZE camera was a Teledyne Princeton Instruments LS-785. Owing to its fast f/2 optical system with proprietary AR-coated lenses for optimum NIR transmission, the LS-785 can achieve up to 4x the throughput of a standard f/4 mirror-based spectrograph. All functions and timing of the CCD camera and the lens-based spectrograph were controlled and coordinated within the experimental setup via Teledyne Princeton Instruments LightField® 64-bit software
Acknowledgment
Teledyne Princeton Instruments wishes to thank Dr. Hideaki Kano (University of Tsukuba, Japan) for his invaluable contributions to this application note.
To learn more about Dr. Hideaki Kano’s research at the University of Tsukuba, please visit: http://www.bk.tsukuba.ac.jp/~CARS/en/index.html
References
- “Interdisciplinary Collaboration on Molecular Fingerprints,” University of Tsukuba (02.12.2015). Accessed online August 2019: https://www.tsukuba.ac.jp/en/people-list/tsukuba-future-036
- Hideaki Kano, Takumi Maruyama, Junko Kano, Yuki Oka, Daiki Kaneta, Tiffany Guerenne, Philippe Leproux, Vincent Couderc, and Masayuki Noguchi, “Ultra-multiplex CARS spectroscopic imaging with 1-millisecond pixel dwell time,” OSA Continuum 2, 1693–1705 (2019). Open access: https://www.osapublishing.org/osac/fulltext.cfm?uri=osac-2-5-1693&id=409415
- “Coherent Anti-Stokes Raman Spectroscopy,” Teledyne Princeton Instruments. Accessed online August 2019: https://www.princetoninstruments.com/applications/coherent-anti-stokes-raman-spectroscopy
- Christoff Krafft, Benjamin Dietzek, and Jürgen Popp, “Raman and CARS microspectroscopy of cells and tissues,” Analyst 134(6), 1046–1057 (2009). doi: 10.1039/b822354h. Epub 2009 Feb 26.
- Eric O. Potma and X. Sunney Xie, “CARS microscopy for biology and medicine,” Optics & Photonics News 15(11), 40–45 (2004). Online: https://doi.org/10.1364/OPN.15.11.000040
- Hideaki Kano and Hiro-o Hamaguchi, “Characterization of supercontinuum generated from a photonic crystal fiber and its application to coherent Raman spectroscopy,” Opt. Online: https://www.osapublishing.org/ol/abstract.cfm?uri=ol-28-23-2360
- Hiroaki Yoneyama, Kazuhiro Sudo, Philippe Leproux, Vincent Couderc, Akihito Inoko, and Hideaki Kano, “Invited Article: CARS molecular fingerprinting using sub-100-ps microchip laser source with fiber amplifier,” APL Photonics 3, 092408 (2018). Open access: https://aip.scitation.org/doi/10.1063/1.5027006
- “A New Dawn for NIR Spectroscopy,” Teledyne Princeton Instruments, 2019. Accessed online August 2019: https://www.princetoninstruments.com/userfiles/files/Tech-Notes/BLAZE-Whitepaper-RevA1.pdf